Safety Instructions
Users of the system require clinical judgment and experience to review and interpret the patient data transmitted.
Notes, Cautions, and Warnings
The types of safety instructions are:
NOTE: Supplementary information to facilitate the operation of the system.
CAUTION: Instructions for avoiding damage to the system.
WARNING: Information may prove hazardous to the safety of a person near the Teladoc Health Patient Access device.
ERROR: An error has occurred.
Safety Symbols
Symbols appearing on the Patient Access device and other equipment are defined in the table below.
| Attention: An alert symbol to notify the user to read the r description of intended use. |   |
| Warning Dangerous Voltage: Touching exposed contacts may cause electrical shock. Safety features designed into the device do not allow exposed live AC contacts until the Patient Access Device is fully engaged with its docking station. When fully engaged, contacts are not accessible. |  |
| Wireless Transmitter Notification: Non-ionizing electromagnetic radiation. This device communicates over the 802.11 ac/a/b/g/n standard for wireless communication. |  |
| Pinch Point: Avoid the labeled pinch point on the rear of the display of your device. Avoid the labeled pinch point between the camera and the first boom joint when the boom is folded to a storage position. Note: The only devices with a boom are the Vantage and Lite 4. |  |
| DC Voltage: This symbol is used to denote the presence of direct current voltage. | 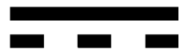 |
| Pinch Point: This label is for the Teladoc Health Vantage and Vici device. The pinch point is located on the left column of the display |  |
| Body Float: Type B. A Type B piece of equipment is one that provides a particular degree of protection against electric shock, particularly regarding allowable leakage current and reliability of the protective earth connection (grounding). |  |
| Consult Operator's Manual: Operating Instructions are contained in a separate instruction manual. |  |
| Do not push or lean: Do not push on cart when it is prevented from lateral movement by an obstruction. |  |
| Force From Above: Observe the overhead Boom when moving or adjusting the position of thePatient Access Device. Overhead objects such as lights can be damaged by unexpected collisions. Note: The only devices with a boom are the Vantage and Lite 4.. |  |
| Tipping Hazard: Do not pull down on the Boom. Fold and lower the Boom to storage position to transport. Note: The only devices with a boom are the Vantage and Lite 4.. |  |
| Do not pull down: or place any heavy objects on the Boom, especially when the Boom is extended. Note: The only devices with a boom are the Vantage and Lite 4.. |  |
|
The Teladoc Health Vita includes a Class II Laser which complies with 21 CFR Chapter 1, Sub-Chapter J. Maximum Laser radiation output is less than one milli-watt at a wavelength of 635 nanometers. CAUTION: Vita users should not direct the laser beam at persons or at reflective surfaces that may cause disturbances. Dazzle, flash-blindness, and afterimages may be caused by a beam from a Class II laser product, particularly under low ambient light conditions. This may have indirect general safety implications resulting from temporary disturbances of vision or from startle reactions. Such visual disturbances could be of particular concern when performing safety-critical operations. |
|
Notes, Cautions, and Warnings
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
Information in ORANGE BOLDFACED TYPE throughout this User Guide should be interpreted in the following context:
NOTE: Provides supplementary information for facilitating operation of the system.
CAUTION: Presents instructions for avoiding damage to the system.
WARNING: Disregarding this information may prove hazardous to the safety of a person near the Teladoc Health Patient Access Device.
Safety Warnings and Cautions
WARNINGS
-
Teladoc Health requires all users to first be trained in the proper use of the HIPAA Resources. The HIPAA Resources stands approximately 5.5 ft. tall (168 cm) and weighs about 180 lb. (82 kg). An untrained operator could potentially bring about a collision, possibly causing damage or injury.
- The Teladoc Health Thermal Camera should not be used as the sole device to diagnose or screen for breast cancer or any other condition. The Teladoc Health Thermal Camera should not be used in place of mammography and is only for use in addition to other diagnostic or screening devices. Incorrect use of the Teladoc Health Thermal Camera carries the risks of a delayed or missed diagnosis, or unnecessary additional tests.
- The Safety and Warnings is not MRI (Magnetic Resonance Imaging) safe, and therefore not MRI compatible. The Safety and Warnings should not be used in locations where the presence of metal is controlled.
-
Use of accessories, transducers and cables other than those specified or provided by the manufacturer of this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of this equipment and result in improper operation.
-
Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the Safety and Warnings, including cables specified by Teladoc Health. Otherwise, degradation of the performance of this equipment could result.
- Flammable Anesthetics: The Safety and Warnings is not suitable for use in the presence of flammable anesthetic mixture with air, or in the presence of a flammable anesthetic mixtures with oxygen or nitrous oxide.
- The Safety and Warnings must be plugged into a grounded “Hospital Grade” electrical outlet to minimize the risk of electrical shock during the battery charging cycle.
- If the power plug of the Safety and Warnings is damaged, the Safety and Warnings can be operated using the onboard battery system. However, this condition should be reported to Technical Service immediately for repair.
- The Safety and Warnings contains a sealed, rechargeable, lead-acid, AGM type battery. The Safety and Warnings should always be plugged in to avoid deep discharge cycles that can shorten the battery’s useful life. Other than keeping the batteries charged by keeping the Safety and Warnings plugged in, no user maintenance of the batteries is required.
- Provide adequate ventilation. The Safety and Warnings may overheat if powered on or plugged in and charging while stored for extended periods in an area without adequate ventilation.
- The power cord may pose a trip hazard if not properly secured prior to moving the Safety and Warnings.
- Avoid manually adjusting the camera's position. Doing so will reset the camera's default "Home", or resting, position. This can only be restored to the original front-facing centered view through the Provider Access Software.
- Leakage current from interconnected electrical equipment may exceed safe levels. In order to maintain patient and user safety, it is important to interconnect only with devices in compliance with IEC 60601-1-1 requirements. It is the responsibility of the user to ensure that any interconnected equipment not supplied by HIPAA Resources maintains compliance with IEC 60601-1-1 requirements.
- Teladoc Health Patient Access Devices are designed to utilize the 802.11 ac/a/b/g/n communication protocol as well as the public Internet in order to achieve their intended purpose. Failures in either of these supporting systems could cause a complete loss of communication between the Provider Access Software and the Patient Access Device. A tested backup method of communication should be available in the event network communication is lost.
- The video images transmitted to and displayed on the Patient Access Device and Teladoc Health Provider Access Software may not contain all of the information in the original scene. Video information from the camera is captured, compressed, transmitted, and redisplayed remotely at a different resolution. As a result, information in the original scene may be lost.
- Color reproduction in the transmitted video is not guaranteed. Color reproduction in a video system is a complicated combination of lighting, cameras, and display technology. It should not be assumed that the colors on the display are an exact replication of the actual colors in the scene.
- Avoid reliance upon video systems for diagnosis and video teleconferencing. Limitations including those outlined above mean video conferencing is not a perfect substitute for in-person interaction with a trained care provider.
- Clinical judgment and experience are required to review and interpret images and information transmitted via the Patient Access Device and Provider Access Software.
- An ESD event may cause the PTZ camera to stop outputting live video. If this should occur, unplug the Safety and Warnings from the wall, and cycle the Safety and Warnings power.
- Do not attempt to open or remove any parts of the Safety and Warnings. To reduce the risk of electric shock, do not remove the cover. There are no user-serviceable components inside. Refer servicing and repair to qualified personnel only.
- Wheels may lock when Safety and Warnings is in motion if lock is engaged accidentally.
- Safety and Warnings may tip forward if pushed with too much force when wheels lock. Please take care when moving to avoid damage or injury.
CAUTIONS:
- Teladoc Health does not support the addition of third party software to an Safety and Warnings. Adding third party software (especially for video conferencing) to the computer can cause the Safety and Warnings to malfunction. Please be advised to check with Technical Service PRIOR to installing any third party software.
- The Safety and Warnings should be plugged in or docked whenever it is possible so it is fully charged and ready for a consult.
- Vici tablet can be removed using a 4mm Hex Allen Wrench. It is recommended to leave the device locked in place.
- There are no user-serviceable components. Refer servicing and repair to qualified personnel only.
- DO NOT IMMERSE the Safety and Warnings. DO NOT ALLOW any cleaning solution inside the Safety and Warnings. Avoid excess solution which may enter the Safety and Warnings through its openings.
- Keep the Safety and Warnings free from moisture and extreme temperatures.
- Teladoc Health has not performed safety and efficacy testing for many peripheral USB devices being used with the Safety and Warnings. Customers must test and validate third-party medical device peripherals for their own use cases and environments
- Ensure external USB devices are disconnected prior to moving the Safety and Warnings.
WARNINGS
- The HIPAA Resources is not MRI safe nor MRI compatible and should only be used in locations where the presence of metal is not controlled.
- Flammable Anesthetics: The HIPAA Resources is not suitable for use in the presence of flammable anesthetic mixture with air, or in the presence of a flammable anesthetic mixture with oxygen or nitrous oxide.
Provider Access Software Cautions
- Avoid driving a Patient Access Device on wet carpets or flooring. It may be difficult to maintain control.
- The Patient Access Device is for indoor use only. Do not drive outside.
- To safely navigate tight spaces it may be necessary to reduce driving speed.
- When using Nudge mode, ensure that contact with the Patient Access Device is only at the bumpers on the base of the Patient Access Device. The Patient Access Device can be damaged if pushed into an obstacle against any part other than the bumpers.
- When experiencing long video/audio delays, InTouch Health recommends driving with caution at a reduced speed.
-
Videos taken through the Provider Access Software are encrypted by default and may only be replayed through the Provider Access Software. Attempting to play videos taken from the Provider Access Software Application outside of the software may require additional software. (Contact Technical Service for more information.)
- For computers with the Stethoscope enhancement feature, a replacement headset must have a frequency response level down to 18 Hz for proper sound quality.
Provider Access Software Warnings
- InTouch Health requires all users to first be trained in the proper use of drivable Patient Access Devices. These Patient Access Devices stand approximately 5.5 ft. tall (168 cm) and weigh between 178 and 220 lb. (81 - 100 kg). An untrained operator could potentially bring about a collision, possibly causing damage or injury.
- The Patient Access Device is designed to utilize the 802.ll communication protocol as well as the public Internet in order to achieve its intended purpose. Failures in either of these supporting systems could cause a complete loss of communication between the Provider Access Software and the Patient Access Device. Consequently, the Patient Access Device should not be utilized in any activities where successful completion of the activity is dependent upon uninterrupted communication between the Patient Access Device and the Provider Access Software.
- A tested backup method of communication should be available in the event network communication is lost.
- Some Patient Access Devices utilizes a Class II Laser which complies with 21 CFR Chapter 1, subchapter j. Maximum Laser radiation output is less than one milliWatt. Refer to the appropriate Patient Access Device User Manual to determine the specific wavelength (color) employed by the laser pointer.
- Dazzle, flash-blindness, and afterimages may be caused by a beam from a Class II laser product, particularly under low ambient light conditions. This may have indirect general safety implications resulting from temporary disturbance of vision or from startle reactions. Such visual disturbances could be of particular concern when performing safety-critical operations. Provider Access Software users should not direct the laser beam at persons or at reflective surfaces that may cause disturbances.
- It is recommended that Provider Access Software access is password protected via operating system's user account control. Only authorized users should have passwords, and users should safeguard passwords according to hospital policies and procedures, thereby preventing untrained users from obtaining access to the system.
- HIPAA regulation compliance requires that the storage of pictures be carefully managed. Consult the hospital’s HIPAA policy director for clarification.
- To avoid inadvertent disclosure of patient-related conversation, we recommend using the microphone mute button when conversing with someone alongside the Provider Access Software during a Remote Presence session.
- Collision avoidance sensors do not serve as a substitute for safe driving. Collision avoidance sensors do not always detect sharp drop-offs such as stairs. Do not attempt to drive the Patient Access Device down stairs and exercise extreme caution while driving near stairs.
- Become oriented with the Patient Access Device and its surroundings before driving. The Patient Access Device should not be driven in extremely close proximity to people (for example, person leaning against Patient Access Device, hugging Patient Access Device, etc.).
- While driving, always look in the direction the Patient Access Device is traveling and both ways at intersections. The driver is responsible for the safe operation of the Patient Access Device—please drive carefully.
-
The video images transmitted to and displayed on the Patient Access Device and Provider Access Software may not contain all of the information in the original scene. Video information from the camera is captured, compressed, transmitted, and redisplayed remotely at a different resolution. As a result information in the original scene may be lost. Color reproduction in the transmitted video is not guaranteed.
- Color reproduction in a video system is a complicated combination of lighting, cameras, and display technology. It should not be assumed that the colors on the display are an exact replication of the actual colors in the scene.
- Clinical judgment and experience are required to review and interpret images and information transmitted via the Patient Access Device and Provider Access Software.
Viewpoint Safety Warnings and Cautions
WARNINGS
-
HIPAA Resources is designed to utilize the 802.ll communication protocol as well as the public Internet in order to achieve their intended purpose. Failures in either of these supporting systems could cause a complete loss of communication between the Teladoc Health Provider Access Software and HIPAA Resources. Consequently, HIPAA Resources should not be utilized in any activities where successful completion of the activity is dependent upon uninterrupted communication between HIPAA Resources and the Teladoc Health Provider Access Software. A tested backup method of communication should always be made available.
-
The video images transmitted to and displayed on the HIPAA Resources and Teladoc Health Provider Access Software may not contain all of the information in the original scene. Video information from the camera is transmitted at either 480p or 720pand redisplayed remotely at a different resolution. As a result, information in the original scene may be lost.
-
Color reproduction in the transmitted video is not guaranteed. Color reproduction in a video system is a complicated combination of lighting, cameras, and display technology. It should not be assumed that the colors on the display are an exact replication of the actual colors in the scene.
-
A tested backup method of communication should be available in the event network communication is lost.
-
Clinical judgment and experience are required to review and interpret images and information transmitted via the Patient Access Device and Provider Access Software.
CAUTIONS
- Teladoc Health does not support the addition of third party software to Teladoc Health HIPAA Resources. Adding third party software (especially for video conferencing) to the computer can cause Teladoc Health HIPAA Resources to malfunction. Please be advised to check with Technical Service PRIOR to installing any third party software.
- The device running Teladoc Health HIPAA Resources should be plugged in whenever it is possible so it is fully charged and ready for a consult.
